Physical Properties:
Appearance : Clear to slight yellow Coloured Liquid
Purity : >95.00% (GC)
Melting Point : NA
Boiling Point : 91°C at 1 mmHg
Storage Temperature : 2-8°C
Conditions to avoid : NA
LOD : NA
Solubility : NA
Density : 1.379 g/mL at 25°C
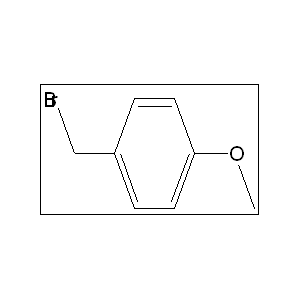
4-Methoxy Benzyl Bromide 2746-25-0, Is a reactive organic compound, typically a colorless to pale yellow liquid, used in organic synthesis as a reagent and intermediate for producing pharmaceuticals and agrochemicals. It features a benzene ring with a methoxy group and a benzyl bromide moiety, making it useful for protecting hydroxyl groups and participating in nucleophilic substitution reactions.
4-Methoxybenzyl bromide is used in pharmaceutical synthesis as an intermediate for compounds like diarylpyrazoles (COX-2 inhibitors for anti-inflammatory drugs) and in organic synthesis for creating other specialty chemicals. It also serves as a reagent for protecting hydroxyl groups, which can be removed with a reagent like DDQ.
Industrial and Research Use
- Pharmaceutical Intermediate:
- It is a key reactant in the synthesis of diarylpyrazoles, which are investigated as COX-2 inhibitors for potential anti-inflammatory and pain-relieving medications.
- It is used in the production of other pharmaceuticals and agrochemicals.
- Research has focused on improving its synthesis to overcome issues with other precursors like 4-methoxybenzyl chloride.
- Protecting Group:
- It is used as a protecting group for hydroxyl (-OH) groups, a common technique in organic synthesis. This protection is reversible, and the group can be removed using a reagent like DDQ (2,3-Dichloro-5,6-dicyano-1,4-benzoquinone).
- Organic Synthesis:
- It is a versatile building block for creating a wide range of organic compounds due to its structure, which allows for further modification.
- It has been used in the synthesis of natural product analogs, such as the recent synthesis of (R)-(–)-argentilactone.
Key Chemical Properties and Research
- Reactivity:
- The methoxy group is electron-donating, which stabilizes the carbocation formed during reactions. This makes 4-methoxybenzyl bromide more reactive than similar compounds with electron-withdrawing groups, such as p-nitrobenzyl bromide.
- Synthesis:
- Various synthesis methods exist, but researchers are working to improve them for better yield and to avoid toxic reagents.
- A common lab synthesis involves the reaction of p-methoxybenzyl alcohol with carbon tetrabromide and triphenylphosphine.
- Role as an Electrophilic Reagent in Organic Synthesis
In many reactions, 4-methoxybenzyl bromide serves as an electrophile, where its benzylic carbon is the site of attack by a nucleophile. This reactivity is fundamental to its use as a protecting group for various functional groups in multistep organic syntheses.
This compound is used to convert carboxylic acids into their corresponding 4-methoxybenzyl (PMB) esters. This transformation is a common strategy for protecting the carboxylic acid functionality during complex syntheses. The reaction typically involves the deprotonation of the carboxylic acid with a base to form a carboxylate salt, which then acts as a nucleophile, attacking the electrophilic benzylic carbon of this compound to form the ester.
Various bases and conditions can be employed for this alkylation. For instance, triethylamine (B128534) is often used in solvents like DMF. In cases involving sensitive substrates or to avoid racemization of chiral centers, the silver salts of carboxylic acids have been alkylated with this compound, reportedly providing higher yields. Another approach utilizes phase-transfer catalysis, which can be advantageous by not requiring strictly anhydrous conditions and often proceeding at lower temperatures. The resulting 4-methoxybenzyl esters are valuable intermediates, as the PMB protecting group can be selectively removed under specific conditions, such as oxidative cleavage with DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) or acidic hydrolysis, often without affecting other sensitive functional groups.
Participation in Grignard Reagent Formation
This compound can participate in the formation of a Grignard reagent. The reaction involves the insertion of magnesium metal into the carbon-bromine bond. This is typically performed in an anhydrous ether solvent, such as tetrahydrofuran (B95107) (THF). The resulting organometallic species is 4-methoxybenzylmagnesium bromide. This reagent is a strong nucleophile and base, useful for forming new carbon-carbon bonds by reacting with electrophiles like aldehydes, ketones, and esters.
It is critical to distinguish between 4-methoxybenzylmagnesium bromide and 4-methoxyphenylmagnesium bromide. The latter, an aryl Grignard reagent, is not prepared from this compound. Instead, 4-methoxyphenylmagnesium bromide is synthesized from the reaction of its corresponding aryl halide, 4-bromoanisole (B123540), with magnesium turnings in an anhydrous ether solvent like THF or diethyl ether.
The reaction is initiated by adding a small amount of an activator, such as iodine, to the magnesium turnings. A solution of 4-bromoanisole in the ether solvent is then added dropwise. The magnesium inserts into the aromatic carbon-bromine bond to yield the Grignard reagent, 4-methoxyphenylmagnesium bromide. This reagent is widely used in organic synthesis to introduce the 4-methoxyphenyl (B3050149) group.
Conjugate Nucleophilic Addition Reactions
While this compound does not directly participate as a nucleophile in conjugate addition reactions, it serves as a highly effective electrophile in tandem reactions that capitalize on the nucleophilic intermediates generated from such additions. The primary role of this compound in this context is the alkylation of enolates formed during a Michael (or 1,4-conjugate) addition.
The process typically involves two key steps:
Michael Addition: A nucleophile (Michael donor), such as a stabilized carbanion derived from a β-dicarbonyl compound, adds to an α,β-unsaturated carbonyl compound (Michael acceptor). This reaction forms a new carbon-carbon bond at the β-position of the acceptor, generating a resonance-stabilized enolate intermediate.
Enolate Trapping: The newly formed enolate is then “trapped” by an electrophile. This compound is an excellent candidate for this step due to its reactive C-Br bond, which is activated by the electron-donating 4-methoxy group. The enolate attacks the benzylic carbon of this compound in a standard SN2-type reaction, displacing the bromide and forming a new C-C bond. This effectively attaches the 4-methoxybenzyl group to the α-position of the original Michael acceptor.
This sequence allows for the controlled, one-pot formation of two new carbon-carbon bonds, introducing significant molecular complexity. The reactivity of the enolate and the choice of the electrophile are critical. In many synthetic strategies, enolates are generated through the deprotonation of a carbonyl precursor with a suitable base; however, their in-situ generation via Michael addition is a powerful alternative. For instance, the reaction of a lithium enolate with an alkyl halide like this compound can proceed with high efficiency, although it may require the presence of additives like hexamethylphosphoramide (B148902) (HMPA) to achieve high yields and desired stereoselectivity depending on the substrate.
This tandem Michael addition-alkylation strategy has been utilized in the stereoselective synthesis of complex molecules. The factors influencing the stereochemical outcome are multifaceted and include the nature of the enolate, the electrophile, and the reaction conditions.
FAQs