Introduction
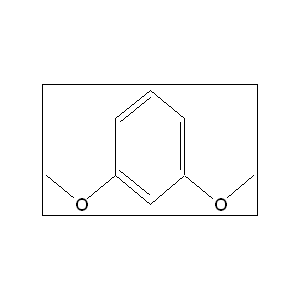
This compound is a valuable organic intermediate in the synthesis of a wide range of compounds, including pharmaceuticals, agrochemicals, and fragrances. Its synthesis from the readily available starting material, resorcinol, is a common transformation in organic chemistry. The most prevalent method for this conversion is the methylation of the hydroxyl groups of resorcinol. This is typically achieved through a Williamson ether synthesis, which involves the reaction of an alkoxide with a primary alkyl halide or another electrophile with a good leaving group. In the case of this compound synthesis, resorcinol is first deprotonated by a base to form the resorcinolate dianion, which then acts as a nucleophile, attacking the methylating agent.
Reaction Mechanism
The synthesis of this compound from resorcinol proceeds via a Williamson ether synthesis, which is a classic SN2 reaction. The overall process can be broken down into two main steps:
- Deprotonation: In the first step, a base, typically a hydroxide such as sodium hydroxide, deprotonates the two acidic phenolic hydroxyl groups of resorcinol. This results in the formation of the resorcinolate dianion, a potent nucleophile.
- Nucleophilic Attack: The resorcinolate dianion then undergoes a nucleophilic attack on the methylating agent, such as dimethyl sulfate. The lone pair of electrons on each oxygen atom attacks the electrophilic methyl group of the dimethyl sulfate, leading to the displacement of the sulfate leaving group in a concerted SN2 fashion. This occurs sequentially for both hydroxyl groups to yield the final product, this compound.
It is important to note that C-alkylation can be a competing side reaction, where the nucleophilic attack occurs at the carbon atoms of the benzene ring rather than the oxygen atoms. However, by carefully selecting the reaction conditions, such as using a weak aqueous alkali, O-alkylation is favored.
FAQs The molecular weight of 1,3-Dimethoxy Benzene (151-10-0) is approximately 138.16 g/mol.